|
By Dr. Burkhard Wehefritz

Monosilane-produced polysilicon (Photo by Schmid Silicon Technology (SST) GmbH)
 Technology Technology
Monosilane
In the 19th century, the German chemists Heinrich Buff and Friedrich Woehler discovered silane as one of the products formed by the reaction of hydrochloric acid on aluminum silicide. Monosilane is toxic and highly flammable when exposed to air even in minimal concentrations. However, the gas has characteristics that make it extremely valuable for our world and environmental protection: Monosilane gas (SiH4) is a basic feedstock for the photovoltaic industry. It is used as an anti-reflective for solar cell coatings, as basis material for thin-film solar cells, flat-screen displays (TFT) and semiconductors for the electronics industry. There are many practical uses, too, like using its water-repellent characteristics and protective functions (e.g., graffiti control) for everyday applications.
Monosilane-Based Polysilicon Production Technology
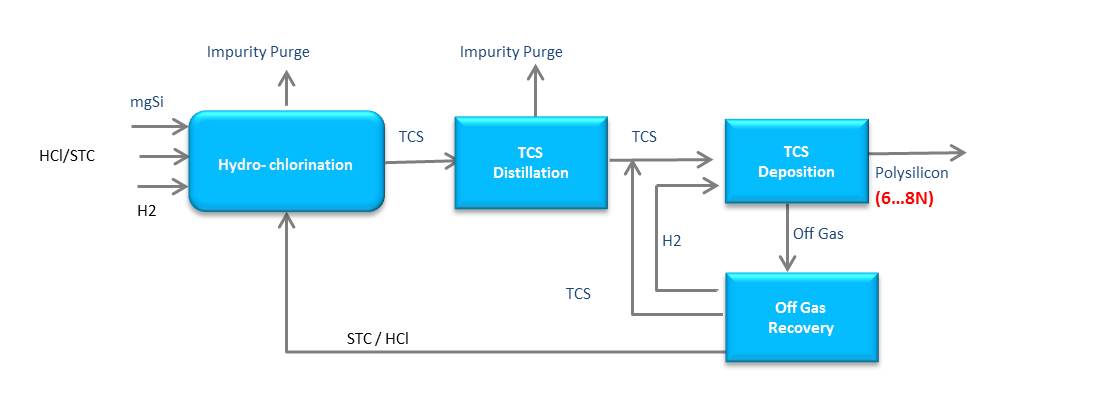
The standard production technique for polysilicon is the TCS (Trichlorosilan)-based production process:
The (modified) Siemens-process:
New:
The monosilane-based polysilicon production process:

Both technologies convert metallurgical silicon into polysilicon; however, the TCS-process has a limited capacity of producing high purity polysilicon necessary for highly efficient solar cells. The monosilane-based process facilitates purification to the degree of electronic-grade high purity polysilicon. In the hydrochlorination in two parallel reactions, both STC (Tetrachlorsilane) and metallurgical silicon respectively are converted into TCS (Trichlorosilane). Then the TCS is fed to a disproportionation reactor, where purified TCS is processed to monosilane gas. The monosilane gas is then directly fed to the CVD reactor, where polysilicon deposition finally occurs on heated slim rods. The monosilane process makes possible an unprecedented silicon conversion rate of 98%.
Safety
Monosilane was first used by the semiconductor industry in 1968. Since then, a series of major accidents contributed to the bad reputation of monosilane gas. In the American semiconductor industry, 36 incidents occurred between 1982 and 1997. Two accidents in Japan, 1989 in a gas cabinet, and 1991 in a laboratory at Osaka University, resulted in three fatalities. Most recent incidents include a silane explosion in Taiwan in 2005 during a cylinder change. The operator was fatally injured.1) This is the reason why some industry players around the globe are hesitant about monosilane even today. There are three major issues that have to be addressed with regard to monosilane: fire and explosion risks, toxicity, and questions of handling and storage.
Fire and Explosion
Silane is a pyrophoric gas that can ignite without any external ignition source with a flammable range of about 1-95% in the air. As experts and silane companies like ASiMI have pointed out in extensive tests, the danger of silane lies in its behaviour when released into air: there can be different kinds of ignition, depending on factors like geometry of release, line pressure of the release and the environment (temperature, humidity etc.) silane is being released into. For example, the oxidation of monosilane is strongly influenced by moisture, since SiH4 is relatively easily hydrolyzed2): SiH4 + 2H2O > SiO2 + 4H2 ; high humidity will reduce the risk of ignition. On the other hand, there is a positive aspect about silane leaks for any plant operating with monosilane: there are no ¡®hidden leaks¡¯. Almost every silane leak is found quickly, because even most tiny silane leaks make a ¡®popping¡¯ sound, or are disclosed by a small flame and a dust cloud instantly. That means, silane leaks are unlikely to accumulate, which prevents vapor clouds that would explode with large impact (as would happen with CH4 or H2). The risk of delayed ignition is higher, the larger the difference between line pressure and ambient pressure is, or when abrupt changes in pressure occur. Then, there is the point of air flow: the most dangerous places for monosilane are small, confined spaces like gas cabinets in fab buildings. The best location for monosilane operating is outdoors, or in large buildings like polysilicon CVD rooms if they have appropriate air flow. Sources of monosilane incidents were in most cases corroded cylinder caps or physically stressed/ uncorrectly used material. Most significant silane industrial accidents have occurred in this context, and not in monosilane production plants. These risks can be managed by good design of equipment and proper handling by trained operators.

Hazardous specification (Source: Schmid Silicon Technology (SST) GmbH)
Toxicity
Silane is not as highly a toxic gas as TCS, STC, DCS, HCl, or Cl2. The byproducts of a silane leak and fire would be SiO2 (dust) and H20 ? all not toxic or harmful gases. In contrast, the byproducts of a TCS, STC, DCS, HCl, or Cl2 release to the atmosphere include highly toxic hydrochloric acid. While such a release of chlorine containing gas can create a toxic cloud that might travel far with the wind and have negative impacts outside plant boundaries, the hazards of monosilane are typically confined to the production facility itself, and provide no risk to the environment. Silane is hard to contain, but not any worse than H2, which is an even smaller and more difficult molecule to contain. H2 leaks are hard to find, they can ignite and burn for a long time undetected since the flame is almost invisible. In fact, monosilane is easier to handle and store than H2. Every polysilicon company knows how to handle H2 safely.
As a matter of fact, the polysilicon industry itself has an excellent record in complying with existing safety codes and regulations when handling hazardous materials. Prevention options, adherence to safety codes and standards, and continuous vigilance guarantee the safe and environmentally friendly nature of the polysilicon industry when handling monosilane.
Outlook
The solar and microelectronics industries need high quality polysilicon and monosilane to remain competitive and investors and poly-producers are looking for low production costs and a high profit margin. No matter whether value or cost-driven motivation or both: innovative technologies like monosilane-based polysilicon production offer the potential to retain competitiveness in a quickly changing market.
=============================================================================
Monosilane Can Be Handled Safely

Schmid Polysilicon Production in Saxony/Germany at night (Photo by Schmid Silicon Technology (SST) GmbH)
SST is an engineering company specialized on the design, production and maintenance of turnkey facilities and components for the polysilicon and monosilane production. The company has been set up in 2006 and developed an alternative production process to the dominant TCS Siemens process for the production of solar-grade polysilicon. Based on proprietary knowledge and technology, it has set up a production facility (SPP, Schmid Polysilicon Production) in Saxony/Germany and has started marketing its turnkey fabs and components to customers around the globe.
The Saxony-based production site, in its beginnings affectionately called pilot plant by its engineers, meanwhile represents an industrial-scale 500mt/a polysilicon plant. Its main purpose, however, remains to be the process development platform for SST as well as the training center for client operators. The production process there is based on SST¡¯s proprietary monosilane technology. SPP announced its First Silicon Out in June of 2011, when according to the press release the polysilicon produced at SPP has reached ¡®electronic grade quality¡¯ (11N or 99.999999999% purity). At the plant, the catalytical disproportionation converts TCS to monosilane (25%) and STC (75%) in one process step.
The experts at SST emphasize that it is an absolutely safe production technology and equipment in the context of any chemical plant. In comparison with other media commonly used to produce polysilicon (e.g. STC, TCS and HCl), monosilane has to be considered as no additional or extraordinary source of risk and can be handled safely under state-of-the-art engineering and plant operation. Hence, a thorough operator training is a very integral part of all of SST¡¯s polysilicon project offerings.
Naturally, SST had to face challenges during building and ramp up of the plant, according to Sales Development Director Dr. Wehefritz, but, in the meantime, all parts of the polysilicon and monosilane production have demonstrated their potential. The fact that the Hydrochlorination step has recently been certified by the TUV organization seems to acknowledge the high expertise of SST`s know-how team that apparently gained, and continues to gain, valuable knowledge associated with the realization of such highly complex projects.
=============================================================================
Dr. Burkhard Wehefritz is Director Sales Development Department at Schmid Silicon Technology (SST) GmbH (www.schmid-silicon.com).
REFERENCES
1) V. Fthenakis, C.C. and W. Chan, National Photovoltaic Environmental Health and Safety Research Center,
Brookhaven National Laboratory, Upton, NY 11973; www.bnl.gov/pv/files/pdf/abs_190.pdf [23.3.2012]
2) A. N. Baratov, L. P. Vogman und L. D. Petrova, Combustion, Explosion, and Shock Waves , Vol. 5, Nr. 4, 407-408, DOI: 10.1007/BF00742094, Explosivity of monosilane-air mixtures
For more information, please send your e-mails to pved@infothe.com.
¨Ï2011 www.interpv.net All rights reserved.
|